What’s gas chromatography and how it works??
Gas chromatography (GC) is an analytical method used to distinguish the compound elements of a sample mix then identify them to ascertain their presence or lack or just how much exists. These compound elements are often organic atoms or molecules. In order to be successful in the analysis of gas chromatography, the components need to have certain things like a weight of below 1250Da, volatile and stable so they don’t worsen in the system. GC is a popular technique across many businesses: for quality management in the production of several products from automobiles to materials to medicines; for study purposes in the evaluation of meteorites to organic goods; and for security from ecological to food into forensics.Gc are regularly hyphenated to GC- MS(MS- Mass Spectrometers) to assist the identification of the elements
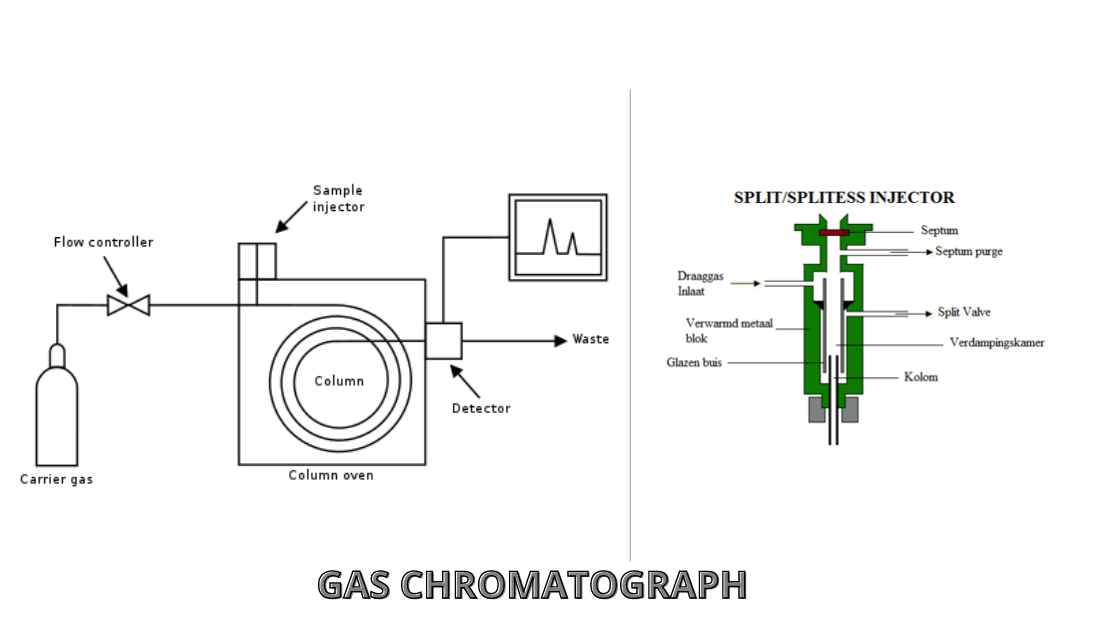
The working principle of Gas chromatography:
The balance for gas chromatography is partitioning, as well as the parts of the sample will probably partition between the 2 stages: the static or stationary phase and the mobile stage. The elements which are with high affinity stays in the column and would elute later, this would lead to a long RT (Retention time).
Affinity for the static phase is determined largely by intermolecular interactions as well as the polarity of the stationary phase could be selected to optimize interactions and therefore the split.
- The split is consequently achieved by partitioning the sample between the gasoline along with a thin coating of a non-volatile liquid stored on a good support.
- A sample comprising the solutes is injected into a heated cube in which it’s instantly vaporized and drifted as a plug in of vapour from the gas flow into the column.
- The solutes are adsorbed in the first stage and then desorbed with a brand-new carrier gas.
- The method is done multiple times in every plate since the sample is transferred toward the socket.
- Every solute will move with its own capability of speed through the pillar.
- Their groups will split into different zones based upon the partition coefficients, and band diffusion.
- Each of these solutes are eluted in a rising order of their kid and move to an attached detector to the exit end of the column. After that, they enroll a couple of signals which would lead to the changes and rates of elution on the recorder as a plot of time versus the composition of the carrier gas stream.
- The look time, width, height, and area of these peaks could be quantified to yield qualitative data.